Please read the following instructions before use.
- Risk category
- Pharmaceuticals Requiring Guidance
- Medicinal effect classification
- Proton pump inhibitor (gastrointestinal medication)
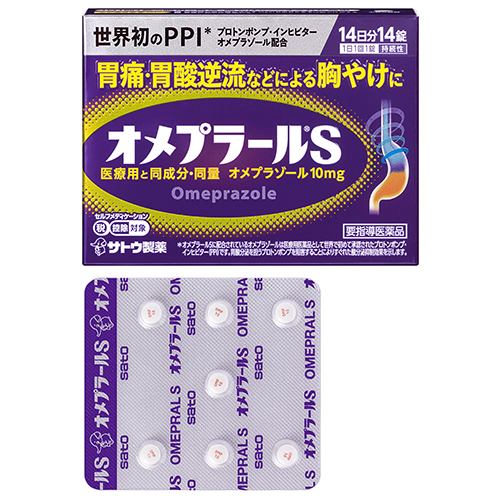
- Product name
- オメプラールS OMEPRAL S Japanese Pharmacopoeia, omeprazole delayed-release tablets
Product information
Precautions
■ When not to use the product
(If you do not follow these instructions, the current symptoms may worsen or adverse reactions/incidents are more likely to occur. )
1. This product should not be taken by the following persons.
(1) Those who have experienced allergic symptoms to this medication or any of its ingredients.
(2) Those who are taking the following drugs:
Rilpivirine hydrochloride
2. This drug should not be taken together with the following drugs:
Other gastrointestinal medications
3. Nursing women should either not take this medicine or stop nursing while taking this medicine.
4. Do not use continuously for more than two weeks including the use of other proton pump inhibitors.
(Consult your physician, since you may be at risk of overlooking a serious digestive disorder. )
■ Consultation
1. The following persons should contact a physician or pharmacist for a consultation before administration.
(1) Patients undergoing medical treatment from a physician.
(2) Pregnant women or women suspected of being pregnant.
(3) The elderly.
(4) Patients who have experienced allergic symptoms associated with drugs, etc.
(5) Those who have been diagnosed with the following:
Liver disease, gastric/duodenal ulcer
(6) Persons with the following symptoms:
Unexplained weight loss, persistent stomach/abdominal pain
2. If the following symptoms are observed after taking this drug, these may be adverse reactions, so immediately discontinue the use of this drug, and show this document to your physician or pharmacist for a consultation.
Related parts: Symptoms
○ Skin: rash/redness, itching, hives
○ Gastrointestinal system: diarrhea, loose stools, constipation, nausea/vomiting, abdominal distension, abdominal pain, stomatitis, glossitis, dry mouth, difficulty swallowing/heartburn/vomiting/white spots in the mouth (suspected candidasis)
○ Neuropsychiatric system: headache, drowsiness, abnormal sensations such as numbness, dizziness, trembling, insomnia, depression,temporary drowsiness, decreased consciousness, loss of consciousness
○ Cardiovascular system: palpitations
○ Urinary system: frequent urination
○ Other: blurred vision, fever, swelling, gynecomastia (male breasts becoming enlarged like a female's), hair loss, general fatigue, joint pain, abnormal taste, menstrual irregularities, muscle pain, sweating, muscle weakness, muscle twitching in the face and limbs, stiffening of the limbs and trembling
The following serious symptoms may occur in rare cases. In such cases, immediately seek medical aid.
Symptom name: Symptoms
○ Shock (anaphylaxis): symptoms, such as itching of skin, urticaria, hoarseness, sneezing, itchy throat, breathing difficulties, palpitations, and clouding of consciousness may occur immediately after take.
○ Oculomucocutaneous syndrome (Stevens-Johnson syndrome) , toxic epidermal necrolysis: hyperthermia, ocular hyperaemia, eye discharge, lip erosion, pain throat, widespread skin rash/redness, etc. may persist or suddenly worsen.
○ Rhabdomyolysis: symptoms may include pain in the muscles of the limbs/shoulders/lower back, numbness, weakness or stiffness in the limbs, general malaise and reddish-brown urine.
○ Hepatic function failure: symptoms, such as fever, itching, rash, jaundice (yellowing of skin and white of eyes) , brown urine, general malaise, loss of appetite, etc. may occur.
○ Kidney disorders: symptoms, such as fever, rash, reduced urinary volume, general oedema, general malaise, arthralgia (painful joints) and diarrhea, etc. may occur.
○ Interstitial pneumonia: shortness and/or difficulties of breath when go upstairs or overwork, sudden dry cough and/or fever and its continuance.
○ Blood disorders: symptoms include sore throat, fever, general fatigue, paleness of the face or the inside of the eyelids, increased bleeding (bleeding gums, nosebleeds, etc.) , bruises (that do not go away when pressed) , dizziness, shortness of breath, yellowing of the whites of the eyes, yellowing of the skin, and dark urine.
○ Visual impairment: difficulty seeing letters and shapes, abnormalities in the field of vision, etc.
○ Hyponatremia: symptoms include convulsions, decreased consciousness, headache, nausea, vomiting, etc.
○ Confusion: symptoms include inattention, incorrect answers to questions, and disorganized behavior.
3. If symptoms do not improve after taking for 3 days, stop taking and consult your physician or pharmacist with this document.
Indication
Stomach pain, heartburn, heavy feeling (this medicine contains a proton pump inhibitor that suppresses the secretion of stomach acid)
Dosage and administration
[Age: Single dose: Number of times per day]
Adults (15 years and older) : 1 tablet: 1 time
Children (under 15 years) : Do not take
● Do not use continuously for more than two weeks.
Precautions of Dosage and Administration
[1] Strictly follow the prescribed dosage and administration.
[2] This medicine dissolves in the intestines. Please take it without chewing or crushing.
[3] Take the medicine once a day at the same time.
[4] Discontinue use once symptoms improve.
[5] How to Dispense Tablets
Using your finger, push the convex portion of the PTP sheet containing capsules to break the aluminum foil on the back.
(Can lead to unforeseen consequences such as piercing the esophageal mucosa if mistakenly swallowed. )
Ingredients, Amount, and Function
In 1 tablet
Omeprazole: 10mg: Suppresses gastric acid secretion by inhibiting the function of the proton pump.
Additive
Sodium lauryl sulfate, cetanol, lactose hydrate, sodium starch glycolate, hydroxypropyl cellulose, magnesium hydroxide, magnesium stearate, hypromellose, synthetic hydrotalcite, titanium oxide, hypromellose phthalate, talc, and carnauba wax.
Precautions for storage and handling Manufacturing and sales company
[1] Store in a cool, low humidity place away from direct sunlight.
[2] Keep out of reach of children.
[3] Do not transfer the medicine to a different container. (It may lead to misuse or alter the quality of the drug. )
[4] Do not take the product after its expiration date.
Customer Service Center
For inquiries about this product, please contact the store where you purchased it or the following:
Customer Service Center [Sato Pharmaceutical Co., Ltd. ]
Tel: 03-5412-7393 Reception hours: 9:00-17:00 (excluding Saturdays, Sundays, and holidays)
Telephone inquiries are only available in Japanese as a language.
Manufacturing and sales company
Sato Pharmaceutical Co., Ltd.
1-5-27 Motoakasaka, Minato-ku, Tokyo
Dosage form
Tablet
Pharmaceutical classification
Pharmaceuticals Requiring Guidance
Version No.
2
Contact details for the Adverse Reaction Relief System
Pharmaceuticals and Medical Devices Agency
https://www.pmda.go.jp/kenkouhigai_camp/index.html
Tel: 0120-149-931 (toll-free)


 Brand site here
Brand site here